Publications
2026
- Impact Factor 5-10Rapid liquid biopsy assessment through gene profiling from the kidney biopsy transport medium: a technical validation and a proof-of-concept pilot studyZiyang Li, Marij J P Welters, Hailiang Mei, Alexis Varin, Baptiste Lamarthée, Aiko P J Vries, Hans J Baelde, and Jesper KersAm J Transplant, Jan 2026
Rapid diagnosis is pivotal in kidney disease for timely and precision therapy. Conventional microscopic and molecular assessments from biopsy tissues rely on extra sample processing, making same-day diagnosis impractical. Therefore, we introduce the biopsy transport medium (BTM), a byproduct of the biopsy tissue storage process that could serve as a source of biomarkers, accelerating the assessment workflow. Biopsies from tumor-free nephrectomy tissues were used to create mimicked BTM, allowing optimization of RNA extraction procedure. RNA yield and integrity were then systematically evaluated prior to downstream analyses. Subsequently, gene expression analysis was performed through multiple techniques: qPCR, RNA sequencing, and NanoString nCounter system. The results showed that storage time (the duration a biopsy is stored in BTM), ranging from 0.5 to 24 hours, did not significantly affect RNA quality and yield. The transcriptomic signals detected in biopsy tissues are largely recapitulated in the corresponding BTM samples. The differential gene expression analysis based on BTM identified rejection-associated profiles, which are aligned with Banff lesion scores. This study confirms BTM’s ability to provide transcriptomic information relevant to the state of the kidney and supports BTM’s potential for same-day molecular diagnosis, especially with tailored qPCR panels for rapid, targeted analysis.
- Impact Factor 10-20In synergy with interferon-gamma, interleukin-17 activates vascular stromal cells towards a pro-inflammatory profile in giant cell arteritisHélène Greigert, André Ramon, Claudie Cladiere, Baptiste Lamarthée, Corentin Richard, Marion Ciudad, Alexis Varin, Noémie Herrmann, Roman Praliaud, Guillaume Brenac, Jérôme Razanamahery, Louis Arnould, Pierre-Henry Gabrielle, Catherine Creuzot-Garcher, Georges Tarris, Laurent Martin, Sylvain Audia, Romain Boidot, Bernard Bonnotte, and Maxime SamsonArthritis Rheumatol, Jan 2026
OBJECTIVES: This study investigated the role of IL-17 in Giant Cell Arteritis (GCA), which has remained uncertain despite previous research suggesting a contribution of Th17 cells to the disease. METHODS: Temporal artery biopsies (TABs) were cultured ex vivo in MATRIGEL\textregistered with IL-17, secukinumab, or control IgG, and subsequently analyzed using bulk RNA-sequencing and RT-qPCR. Positive-TABs with GCA features were compared to negative-TABs or used to obtain in vitro cultures of myofibroblasts (MFs). Confocal microscopy analyzed IL-17 receptor expression. MFs and peripheral blood mononuclear cells co-cultures were used to study T-cell polarization. RESULTS: Transcriptomic analysis showed that secukinumab treatment of positive TABs reduced expression of genes linked to vascular inflammation, notably IL6. RT-qPCR analysis confirmed that secukinumab decreased mRNA encoding IL-6, CCL20, and GM-CSF in positive-TABs, while IL-17 upregulated them in negative-TABs. No changes were observed regarding the expression of genes related to vascular remodeling. IL-17 receptor chains were expressed on MFs, and their expression was enhanced by IFN-γ. RT-qPCR and Luminex\textregistered analyses confirmed IL-17-driven upregulation of IL-6, CCL20, CCL2, GM-CSF, and VEGF in MFs, which was reversed by secukinumab. Addition of IFN-γto the culture increased the expression level of IL-17 receptor chains, resulting in a synergistic effect. Additionally, IL-17 pre-treated MFs promoted Th17 polarization. CONCLUSIONS: IL-17 exacerbates vascular inflammation in GCA by activating MFs and synergizing with IFN-γto increase production of pro-inflammatory cytokines (IL-6, GM-CSF), chemokines (CCL20, CCL2), and angiogenic factors (VEGF, indicating that IL-17 is a key contributor to disease pathogenesis.
- Impact Factor 10-20
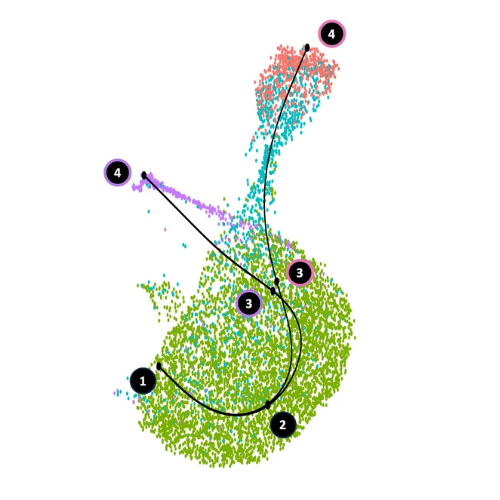
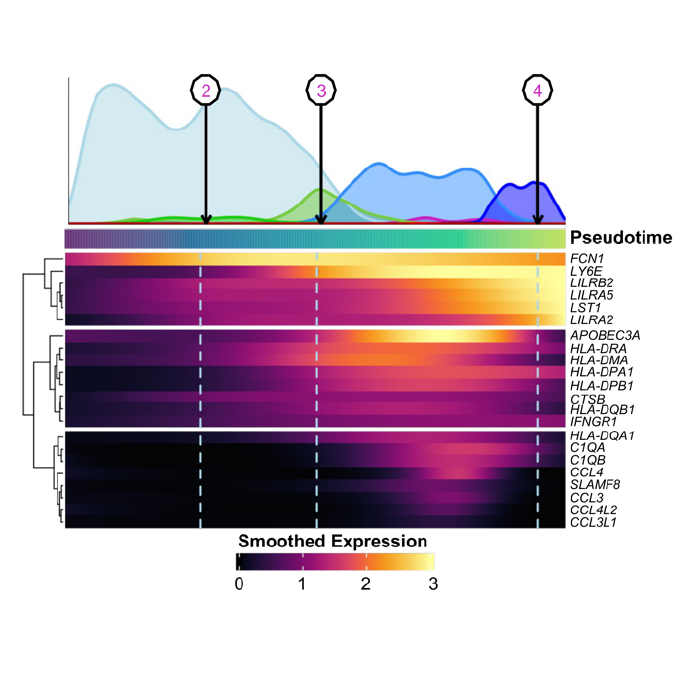 A Subset of Pro-inflammatory CXCL10+ LILRB2+ Macrophages Derives From Recipient Monocytes and Drives Renal Allograft RejectionAlexis Varin*, Jovanne Palvair*, Lennie Messager*, Jamal Bamoulid, Jasper Callemeyn, Mélanie Chaintreuil, Ludivine Dal Zuffo, Didier Ducloux, Imane Farhat, Mathieu Legendre, Laurent Martin, Florian Renosi, Xavier Roussel, Thibaut Vaulet, Maarten Naesens, Claire Tinel†, and Baptiste Lamarthée†Adv Sci, Jan 2026
A Subset of Pro-inflammatory CXCL10+ LILRB2+ Macrophages Derives From Recipient Monocytes and Drives Renal Allograft RejectionAlexis Varin*, Jovanne Palvair*, Lennie Messager*, Jamal Bamoulid, Jasper Callemeyn, Mélanie Chaintreuil, Ludivine Dal Zuffo, Didier Ducloux, Imane Farhat, Mathieu Legendre, Laurent Martin, Florian Renosi, Xavier Roussel, Thibaut Vaulet, Maarten Naesens, Claire Tinel†, and Baptiste Lamarthée†Adv Sci, Jan 2026In solid organ transplantation, monocytes and macrophages play a cross-cutting role in the rejection process, irrespective of the transplanted tissue and the type of rejection. Here, we integrated multiple single-cell assays (>150,000 cells) with a broad spectrum of blood-derived and renal allograft-derived cells. We observed 6 myeloid cell trajectories enriched in the allograft during rejection, ranging from circulating CD14+ monocytes to differentiated macrophages in the kidney, with one trajectory culminating in a pro-inflammatory macrophage expressing CXCL9 and CXCL10. By analyzing over 850 biopsies using deconvolution, we report that they are absent in pre-transplant allografts, while these CXCL10+ macrophages are the immune cells most associated with inflammation during acute rejection. Furthermore, a survival study of over 500 biopsies indicates that they increase the risk of graft loss independently of other immune cells. CXCL10+ macrophages differentiate from recipient monocytes, and we have identified 6 major genes associated with their differentiation, including LILRB2. In vitro, mimicking allogenic activation of blood monocytes via the CD47/SIRP-a axis induced overexpression of LILRB2, suggesting that CXCL10+ macrophages are activated by this pathway. Finally, we show that macrophages overexpressing LILRB2 induce the proliferation of autologous T lymphocytes. Altogether, the present study provides further insight into the pro-inflammatory axes of recipient-derived monocytes/macrophages, and suggests LILRB2 as a therapeutic target.
2025
- Impact Factor 10-20
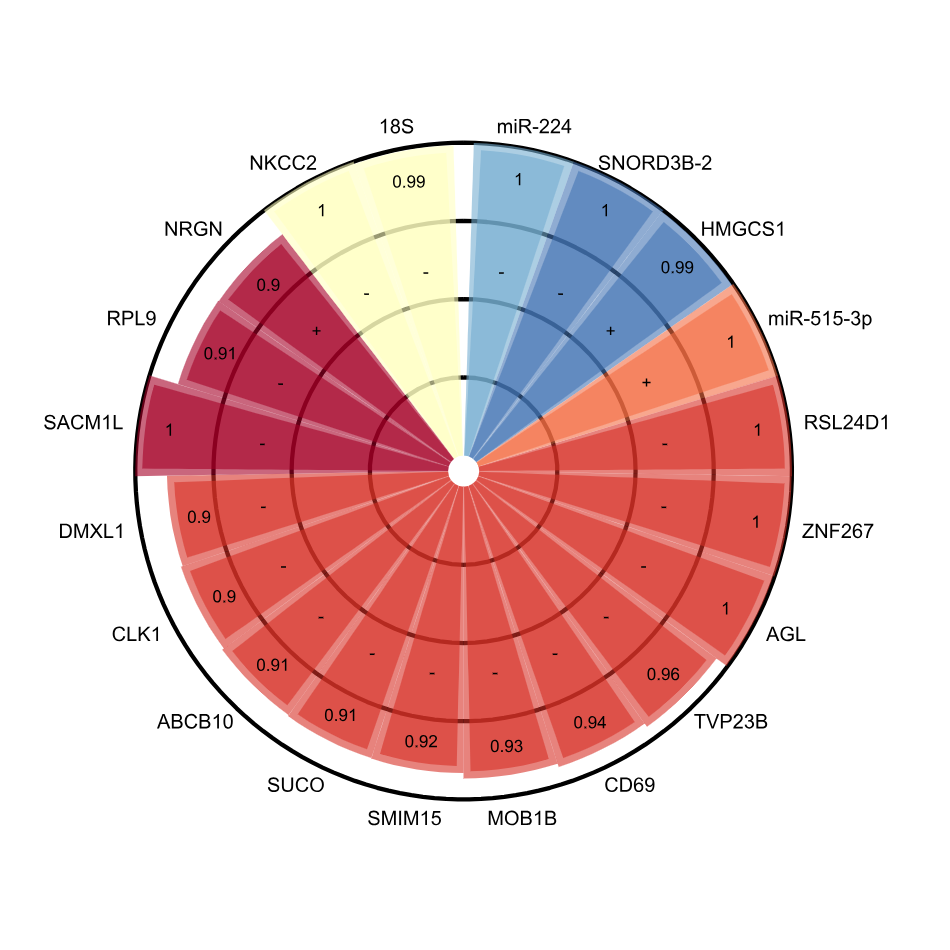 Multi-omic factor analyses uncovered cross-compartment complexity of biological processes in kidney transplantationClaire Tinel*, Alexis Varin*, Dany Anglicheau, Jasper Callemeyn, Jetty De Loor, Wilfried Gwinner, Pierre Marquet, Marion Rabant, Virginia Sauvaget, Elisabet Van Loon, Maarten Naesens†, and Baptiste Lamarthée†Kidney Int, Nov 2025
Multi-omic factor analyses uncovered cross-compartment complexity of biological processes in kidney transplantationClaire Tinel*, Alexis Varin*, Dany Anglicheau, Jasper Callemeyn, Jetty De Loor, Wilfried Gwinner, Pierre Marquet, Marion Rabant, Virginia Sauvaget, Elisabet Van Loon, Maarten Naesens†, and Baptiste Lamarthée†Kidney Int, Nov 2025INTRODUCTION: Understanding the complexity of pathways involved in allograft injuries after solid organ transplantation is essential for precise definitions of rejection subtypes and improved overall outcomes. High throughput technologies and the recently available computational methods make it now possible to address such complex biological questions. Here, we performed a unique compilation of six omics datasets (170,000 variables) from a multi-center study encompassing 131 kidney transplant recipients. METHODS: Using multi-omics factor analysis (MOFA), we investigated sources of variability in patient blood, urine and their allograft at the epigenetic and transcriptomic levels. RESULTS: Integrating the different omics layers, MOFA delimited eight hidden factors in an unsupervised manner. We identified specific factors that reflect allograft rejection and their multicellular complex immune profiles, complement activation, monocyte crosstalk, or immune modifications associated with induction treatment. CONCLUSIONS: These cross-compartment large datasets translated into an understandable biological picture provide a new framework to solve complex biological questions, not unique to transplant medicine.
- Preprint
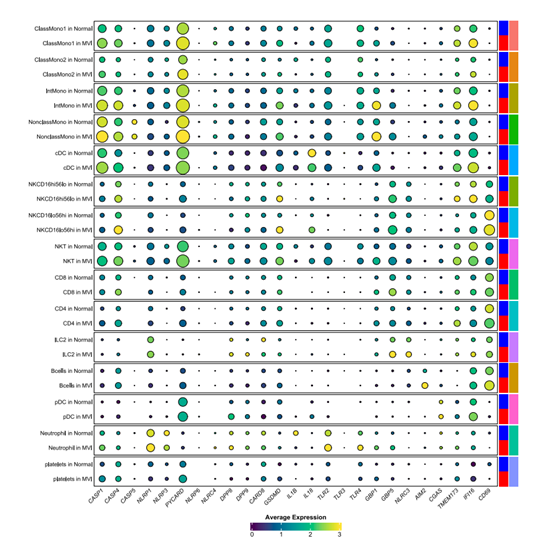 Inflammasome Profiling in Human Natural Killer Cells under Pro-inflammatory Stimuli and Solid Organ TransplantationAntonio Astorga-Gamaza, Inés Muela-Zarzuela, Juan Miguel Suárez-Rivero, Alexis Varin, Maarten Naesens, Alessandra Tammaro, Jesper Kers, Juan López-Pérez, Raquel Varga-Martı́nez, Auxiliadora Mazuecos, Baptiste Lamarthée, and Mario D. CorderobioRxiv, Oct 2025
Inflammasome Profiling in Human Natural Killer Cells under Pro-inflammatory Stimuli and Solid Organ TransplantationAntonio Astorga-Gamaza, Inés Muela-Zarzuela, Juan Miguel Suárez-Rivero, Alexis Varin, Maarten Naesens, Alessandra Tammaro, Jesper Kers, Juan López-Pérez, Raquel Varga-Martı́nez, Auxiliadora Mazuecos, Baptiste Lamarthée, and Mario D. CorderobioRxiv, Oct 2025Innate immunity relies on inflammasomes as key mediators of host defense, orchestrating the release of pro-inflammatory cytokines and triggering pyroptotic cell death in response to harmful stimuli. Although inflammasome activity has been extensively studied in myeloid cells, its role in natural killer (NK) cells remains underexplored. This study demonstrates that human primary NK cells can functionally activate inflammasomes both in vitro and in vivo, including in patients undergoing organ transplantation. Ex vivo stimulation with nigericin and the dipeptidyl peptidases (DPP) inhibitor Talabostat (Val-boroPro) induces pyroptotic cell death in a subset of NK cells. This is marked by the cleavage and activation of gasdermin D, a lytic pore-forming protein essential for pyroptosis. Accompanying gasdermin D activation, significant levels of lactate dehydrogenase (LDH) and residual amounts of interleukin-18 (IL-18) are released. The detection of activated caspase-4 further indicates that these processes are mediated through non-canonical inflammasome pathways in NK cells. Notably, CD56dim and CD56bright NK cell subsets exhibit distinct responses to pro-inflammatory stimulation. In patients with renal dysfunction, sustained inflammasome activation, particularly involving NLRP1 and NLRP3, is observed in NK cells, with a shift toward a more pro-inflammatory phenotype following kidney transplantation. Single-cell RNA sequencing analyses further reveal persistently elevated expression of caspase-4 and gasdermin-D in transplant recipients experiencing rejection and microvascular inflammation. These findings highlight the underappreciated role of NK cells in inflammasome-driven inflammation, underscoring their importance in both basic research and clinical contexts.
- Impact Factor < 5
 Mature CD209+CD83+CCR7+ dendritic cells infiltrate the arterial wall in giant cell arteritis and derive from in-situ monocyte differentiationAndré Ramon, Hélène Greigert, Baptiste Lamarthée, Corentin Richard, Alexis Varin, Claudie Cladière, Coraline Genet, Marion Ciudad, Noémie Klopfenstein, Roman Praliaud, Guillaume Brenac, Georges Tarris, Laurent Martin, Louis Arnould, Pierre-Henry Gabrielle, Catherine Creuzot Garcher, Paul Ornetti, Sylvain Audia, Romain Boidot, Jean-Francis Maillefert, Bernard Bonnotte, and Maxime SamsonSci Rep, Oct 2025
Mature CD209+CD83+CCR7+ dendritic cells infiltrate the arterial wall in giant cell arteritis and derive from in-situ monocyte differentiationAndré Ramon, Hélène Greigert, Baptiste Lamarthée, Corentin Richard, Alexis Varin, Claudie Cladière, Coraline Genet, Marion Ciudad, Noémie Klopfenstein, Roman Praliaud, Guillaume Brenac, Georges Tarris, Laurent Martin, Louis Arnould, Pierre-Henry Gabrielle, Catherine Creuzot Garcher, Paul Ornetti, Sylvain Audia, Romain Boidot, Jean-Francis Maillefert, Bernard Bonnotte, and Maxime SamsonSci Rep, Oct 2025This study aimed to characterize arterial dendritic cells (DCs) in polymyalgia rheumatica (PMR) and giant cell arteritis (GCA). Bulk RNA-sequencing, RT-PCR and immunofluorescence analyses were performed from temporal arteries from GCA, PMR and control patients. Public single-cell RNA-seq (scRNA-seq) data on Peripheral Blood Mononuclear Cells (PBMCs) were analyzed from three GCA and three control patients. Bulk RNA-Seq and RT-PCR analyses demonstrated a high level of expression of DC lineage markers (CD209), DC maturation markers (CD83, CCR7) and chemokines associated with DC maturation in GCA arteries. The level of expression of DC lineage and DC maturation associated genes was significantly lower in PMR than in GCA arteries and similar between PMR and control arteries. GCA arteries expressed high levels of GM-CSF and IFNG mRNA. ScRNA-seq analysis of GCA PBMCs demonstrated high expression of IFNGR and CSF2R by classical monocytes and cultures of CD14(+) monocytes with GM-CSF and IFN-γwere able to promote their differentiation into monocyte derived-DCs (mo-DCs). This work provides evidence that mo-DCs infiltrate GCA lesions and could be generated under the influence of GM-CSF and IFN-γfrom monocytes infiltrating the arterial wall. Mo-DCs could play an important role in GCA pathogenesis and be targeted by GM-CSF and/or IFN-γinhibitors.
- Impact Factor 5-10HUMANIN produced by human efferocytic macrophages promotes the resolution of inflammationMélissa Maraux, Mathieu Vetter, Ludivine Dal Zuffo, Francis Bonnefoy, Audrey Wetzel, Alexis Varin, Baptiste Lamarthée, Olivier Tassy, Didier Ducloux, Philippe Saas, and Thomas CherrierCell Death Dis, Aug 2025
Elimination of apoptotic neutrophils by macrophages, a process called efferocytosis, is a critical step in the resolution of inflammation. Efferocytosis induces the reprogramming of macrophages towards a pro-resolving phenotype and triggers the secretion of pro-resolving factors. While mouse efferocytic macrophages are well-described, less is known about human efferocytic macrophages. Here, using RNA sequencing analysis of three different types of in vitro-derived human efferocytic macrophages, we observed a common modulation of mitochondrial metabolism-related genes in human M0, M1, and M2a-like macrophages, thus correlating with some previous results obtained in other non-human models. These results led us to identify for the first time some particular genes regulated in humans like PLIN5 and MTLN. We also shed light on a mitochondrial gene (MT-RNR2) coding a secreted factor called HUMANIN. Mainly known for its antioxidant and neuroprotective effects, we found that HUMANIN was also associated with pro-resolving properties in human and mouse models. Indeed, HUMANIN was produced early during the resolution of inflammation in an acute peritonitis mouse model. Preventive HUMANIN administration in this model reduced leukocyte infiltration and pro-inflammatory cytokine secretion. These anti-inflammatory properties were accompanied by the early acquisition of a CD11b(low) non-efferocytic phenotype by mouse macrophages and by an enhanced expression of pro-resolving genes including Alox15 and Retnla. The ability of HUMANIN to dampen pro-inflammatory cytokine secretion was also confirmed in primary human neutrophils. Finally, HUMANIN was also detected in gingival crevicular fluids of patients suffering from periodontitis after the onset of inflammation, suggesting a role of HUMANIN in the control of inflammation. Overall, our data shed light on new aspects of efferocytosis in humans and identify the pro-resolving potential of HUMANIN. This illustrates its prospective therapeutic interest in inflammatory disorders.
- DatasetKidney Transplant Biopsy-derived signature matrix of 18 cell phenotypes (KTB18) for deconvolution using the CIBERSORTx algorithmAlexis Varin, Baptiste Lamarthée, Jasper Callemeyn, Yannick Van Herck, Asier Antoranz, Dany Anglicheau, Patrick Boada, Jan Ulrich Becker, Tim Debyser, Frederik De Smet, Katrien De Vusser, Maëva Eloudzeri, Amelie Franken, Wilfried Gwinner, Priyanka Koshy, Dirk Kuypers, Diether Lambrechts, Pierre Marquet, Virginie Mathias, Marion Rabant, Minnie M. Sarwal, Aleksandar Senev, Tara K. Sigdel, Ben Sprangers, Olivier Thaunat, Claire Tinel, Thomas Van Brussel, Amaryllis Van Craenenbroeck, Elisabet Van Loon, Thibaut Vaulet, Francesca M. Bosisio, and Maarten NaesensJul 2025
- Impact Factor 5-10Active immunologic participation and metabolic shutdown of kidney structural cells during kidney transplant rejectionElisabet Van Loon*, Baptiste Lamarthée*, Jasper Callemeyn, Imane Farhat, Priyanka Koshy, Dany Anglicheau, Pietro Cippà, Amelie Franken, Wilfried Gwinner, Dirk Kuypers, Pierre Marquet, Anna Rinaldi, Claire Tinel, Thomas Van Brussel, Amaryllis Van Craenenbroeck, Alexis Varin, Thibaut Vaulet, Diether Lambrechts, and Maarten NaesensAm J Transplant, Mar 2025
Contrary to immune cells, the response of the kidney structural cells in rejection is less established. We performed single-cell RNA sequencing of 18 kidney transplant biopsies from 14 recipients. Single-cell RNA sequencing identified cells from the major compartments of the kidney, next to infiltrated immune cells. Endothelial cells from the glomerulus, peritubular capillaries, and vasa recta showed upregulation of class I and II human leukocyte antigen genes, adhesion molecules, cytokines, and chemokines, suggesting active participation in the alloimmune process, with compartment-specific differences. Epithelial cells including proximal tubular, loop of Henle, and collecting duct cells, also showed increased expression of immune genes. Strikingly, in proximal tubule cells, a strong downregulation of energy metabolism upon inflammation was observed. There was a large overlap between the cell-specific expression changes upon alloimmune inflammation and those observed in 2 large microarray biopsy cohorts. In conclusion, the kidney structural cells, being the main target of the alloimmune process, appear to actively contribute herein, enhancing the damaging effects of the infiltrating immune cells. In epithelial cells, a profound shutdown of metabolism was seen upon inflammation, which is associated with poor kidney function. These observations highlight the critical role of the graft in triggering and sustaining rejection after transplantation.
- DatasetBIOMARGIN study step 2 & 3: RT-PCR on urine samplesAlexis Varin, Claire Tinel, Dany Anglicheau, Jasper Callemeyn, Jetty De Loor, Wilfried Gwinner, Pierre Marquet, Marion Rabant, Virginia Sauvaget, Elisabet Van Loon, Baptiste Lamarthée, and Maarten NaesensJan 2025
- DatasetBIOMARGIN study step 1: RT-PCR on urine samplesAlexis Varin, Claire Tinel, Dany Anglicheau, Jasper Callemeyn, Jetty De Loor, Wilfried Gwinner, Pierre Marquet, Marion Rabant, Virginia Sauvaget, Elisabet Van Loon, Baptiste Lamarthée, and Maarten NaesensJan 2025
- Impact Factor 10-20Impaired unsaturated fatty acid elongation alters mitochondrial function and accelerates metabolic dysfunction-associated steatohepatitis progressionAdrien Vouilloz, Thibaut Bourgeois, Marc Diedisheim, Thomas Pilot, Antoine Jalil, Naig Le Guern, Victoria Bergas, Noéline Rohmer, Florence Castelli, Damien Leleu, Alexis Varin, Jean-Paul Pais Barros, Pascal Degrace, Mickael Rialland, Camille Blériot, Nicolas Venteclef, Charles Thomas, and David MassonMetabolism, Jan 2025
BACKGROUND AND AIMS: Although qualitative and quantitative alterations in liver Polyunsaturated Fatty Acids (PUFAs) are observed in MASH in humans, a causal relationship of PUFAs biosynthetic pathways is yet to be clarified. ELOVL5, an essential enzyme in PUFA elongation regulates hepatic triglyceride metabolism. Nonetheless, the long-term consequences of elongase disruption, particularly in murine models of MASH, have not been evaluated. APPROACH & RESULTS: In humans, transcriptomic data indicated that PUFAs biosynthesis enzymes and notably ELOVL5 were induced during MASH progression. Moreover, gene module association determination revealed that ELOVL5 expression was associated with mitochondrial function in both humans and mice. WT and Elovl5-deficient mice were fed a high-fat, high-sucrose (HF/HS) diet for four months. Elovl5 deficiency led to limited systemic metabolic alterations but significant hepatic phenotype was observed in Elovl5-/- mice after the HF/HS diet, including hepatomegaly, pronounced macrovesicular and microvesicular steatosis, hepatocyte ballooning, immune cell infiltration, and fibrosis. Lipid analysis confirmed hepatic triglyceride accumulation and a reshaping of FA profile. Transcriptomic analysis indicated significant upregulation of genes involved in immune cell recruitment and fibrosis, and downregulation of genes involved in oxidative phosphorylation in Elovl5-/- mice. Alterations of FA oxidation and energy metabolism were confirmed by non-targeted metabolomic approach. Analysis of mitochondrial function in Elovl5-/- mice showed morphological alterations, qualitative cardiolipin changes with an enrichment in species containing shorter unsaturated FAs, and decreased activity of I and III respiratory chain complexes. CONCLUSION: Enhanced susceptibility to diet-induced MASH and fibrosis in Elovl5-/- mice is intricately associated with disruptions in mitochondrial homeostasis, stemming from a profound reshaping of mitochondrial lipids, notably cardiolipins.
2024
2023
- Impact Factor 5-10Amelioration of experimental autoimmune encephalomyelitis by in vivo reprogramming of macrophages using pro-resolving factorsThierry Gauthier, Omayra Martin-Rodriguez, Cécile Chagué, Anna Daoui, Adam Ceroi, Alexis Varin, Francis Bonnefoy, Séverine Valmary-Degano, Mélanie Couturier, Susanne Behlke, Philippe Saas, Pierre-François Cartron, and Sylvain PerrucheJ Neuroinflammation, Dec 2023
BACKGROUND: Reinstating inflammation resolution represents an innovative concept to regain inflammation control in diseases marked by chronic inflammation. While most therapeutics target inflammatory molecules and inflammatory effector cells and mediators, targeting macrophages to initiate inflammation resolution to control neuroinflammation has not yet been attempted. Resolution-phase macrophages are critical in the resolution process to regain tissue homeostasis, and are programmed through the presence and elimination of apoptotic leukocytes. Hence, inducing resolution-phase macrophages might represent an innovative therapeutic approach to control and terminate dysregulated neuroinflammation. METHODS: Here, we investigated if the factors released by in vitro induced resolution-phase macrophages (their secretome) are able to therapeutically reprogram macrophages to control neuroinflammation in the model of experimental autoimmune encephalomyelitis (EAE). RESULTS: We found that injection of the pro-resolutive secretome reduced demyelination and decreased inflammatory cell infiltration in the CNS, notably through the in vivo reprogramming of macrophages at the epigenetic level. Adoptive transfer experiments with in vivo or in vitro reprogrammed macrophages using such pro-resolutive secretome confirmed the stability and transferability of this acquired therapeutic activity. CONCLUSIONS: Overall, our data confirm the therapeutic activity of a pro-resolution secretome in the treatment of ongoing CNS inflammation, via the epigenetic reprogramming of macrophages and open with that a new therapeutic avenue for diseases marked by neuroinflammation.
- Impact Factor 5-10Resolved Psoriasis with Abundant Oleic Acid in Stratum Corneum Exhibits Lower T-Cell-Driven IL-17 SignatureYasmin El Mahi, Alexis Varin, Mathieu Vetter, Ludivine Dal Zuffo, Loı̈c Mazzeo, Jean-Paul Pais De Barros, François Aubin, Philippe Saas, and Irène Gallais SérézalJ Invest Dermatol, Nov 2023
Relapses of psoriasis involve T cells that stem and survive in the skin. Inherited from previous flares, the tissue-resident memory T cells are epidermal IL-17-producing CD8(+) and IL-22-producing CD4(+) T cells. Because the capacity of resident memory T cells to take in fatty acids is essential for their residence and function, the surface composition of fatty acids may affect underlying T-cell populations. In patients treated with biologics, we used gas chromatography/mass spectrometry to decipher the fatty acid composition in both resolved and nonlesional sites. Skin T cells were activated by OKT-3 in explants from the same body sites to perform bulk transcriptomic analysis (Nanostring). The fatty acid composition differed between skin from healthy donors and normal-looking skin of patients with psoriasis but not further between nonlesional and resolved skin. Patients in whom the resolved skin was rich in oleic acid had lower T-cell-driven IL-17 epidermal transcriptomic signature upon activation of T cells in skin explants. The skin lipid composition is linked with the functions of the underlying epidermal T cells. Testing the modulating effect of custom fatty acids on skin resident T cells could help with coming closer to disease oblivion in inflammatory skin diseases.
2022
- Impact Factor < 5Profiling of lipid mediators in atherosclerotic carotid plaques from type 2 diabetic and non-diabetic patientsLouise Ménégaut*, Aline Laubriet*, Valentin Crespy, Maxime Nguyen, Jean-Michel Petit, Georges Tarris, Thomas Pilot, Alexis Varin, Hélène Choubley, Victoria Bergas, Jean-Paul Pais Barros, Charles Thomas, Eric Steinmetz, and David MassonProstaglandins Leukot Essent Fatty Acids, Sep 2022
BACKGROUND AND AIMS: Diabetes is associated with an accelerated development of atherosclerosis. Specific mechanisms related to diabetes and hyperglycemia may play a role in this process. In particular, alterations of arachidonic acid (AA) metabolism have been reported. Our main goal was to investigate for differences in the concentration of LTB4 and RvD1 as well as selected cyclooxygenase-derived mediators in carotid plaques from diabetic and non-diabetic patients. We also aimed to analyze the relationship between omega 6 and omega 3 Poly-Unsaturated Fatty acids (PUFAs) content in the plaques and the concentrations of these lipid mediators. METHODS: 29 type 2 diabetic patients and 30 control patients admitted for surgical treatment of carotid stenosis were enrolled in the present study. Carotid plaques were harvested for in-depth lipidomic profiling. RESULTS: No differences for LTB4 or other lipid mediators were observed between diabetic and non-diabetic patients. RvD1 levels were below the threshold of quantification in most of the samples. A significant correlation was found between LTB4 and 5(S)-HETE levels. Omega 3 enrichment was not significantly different between control and diabetic plaques. There was a negative correlation between DHA/AA ratio and the level of 5(S)-HETE while there was a positive association with TXB2 and PGD2 concentrations. CONCLUSION-PERSPECTIVES: Our results does not support the hypothesis of a specific involvement of LTB4 or COX-derived mediators in diabetic atherosclerosis. The relationship between DHA enrichment and the concentrations of specific inflammatory mediators within the plaque is of interest and will need to be confirmed in larger studies.
2017
- Impact Factor < 5Recent insights into the implications of metabolism in plasmacytoid dendritic cell innate functions: Potential ways to control these functionsPhilippe Saas, Alexis Varin, Sylvain Perruche, and Adam CeroiF1000Res., Apr 2017
There are more and more data concerning the role of cellular metabolism in innate immune cells, such as macrophages or conventional dendritic cells. However, few data are available currently concerning plasmacytoid dendritic cells (PDC), another type of innate immune cells. These cells are the main type I interferon (IFN) producing cells, but they also secrete other pro-inflammatory cytokines (e.g., tumor necrosis factor or interleukin [IL]-6) or immunomodulatory factors (e.g., IL-10 or transforming growth factor-β). Through these functions, PDC participate in antimicrobial responses or maintenance of immune tolerance, and have been implicated in the pathophysiology of several autoimmune diseases, as well as in tumor immune escape mechanisms. Recent data support the idea that the glycolytic pathway (or glycolysis), as well as lipid metabolism (including both cholesterol and fatty acid metabolism) may impact some innate immune functions of PDC or may be involved in these functions after Toll-like receptor (TLR) 7/9 triggering. The kinetics of glycolysis after TLR7/9 triggering may differ between human and murine PDC. In mouse PDC, metabolism changes promoted by TLR7/9 activation may depend on an autocrine/paracrine loop, implicating type I IFN and its receptor IFNAR. This could explain a delayed glycolysis in mouse PDC. Moreover, PDC functions can be modulated by the metabolism of cholesterol and fatty acids. This may occur via the production of lipid ligands that activate nuclear receptors (e.g., liver X receptor [LXR]) in PDC or through limiting intracellular cholesterol pool size (by statin or LXR agonist treatment) in these cells. Finally, lipid-activated nuclear receptors (i.e., LXR or peroxisome proliferator activated receptor) may also directly interact with pro-inflammatory transcription factors, such as NF-\kappaB. Here, we discuss how glycolysis and lipid metabolism may modulate PDC functions and how this may be harnessed in pathological situations where PDC play a detrimental role.
2015
- Impact Factor 5-10Liver X receptor activation promotes polyunsaturated fatty acid synthesis in macrophages: relevance in the context of atherosclerosisAlexis Varin, Charles Thomas, Minako Ishibashi, Louise Ménégaut, Thomas Gautier, Amalia Trousson, Victoria Bergas, Jean Paul Pais Barros, Michel Narce, Jean Marc A Lobaccaro, Laurent Lagrost, and David MassonArterioscler. Thromb. Vasc. Biol., Jun 2015
OBJECTIVE: Liver X receptors (LXRs) modulate cholesterol and fatty acid homeostasis as well as inflammation. This study aims to decipher the role of LXRs in the regulation of polyunsaturated fatty acid (PUFA) synthesis in macrophages in the context of atherosclerosis. APPROACH AND RESULTS: Transcriptomic analysis in human monocytes and macrophages was used to identify putative LXR target genes among enzymes involved in PUFA biosynthesis. In parallel, the consequences of LXR activation or LXR invalidation on PUFA synthesis and distribution were determined. Finally, we investigated the impact of LXR activation on PUFA metabolism in vivo in apolipoprotein E-deficient mice. mRNA levels of acyl-CoA synthase long-chain family member 3, fatty acid desaturases 1 and 2, and fatty acid elongase 5 were significantly increased in human macrophages after LXR agonist treatment, involving both direct and sterol responsive element binding protein-1-dependent mechanisms. Subsequently, pharmacological LXR agonist increased long chain PUFA synthesis and enhanced arachidonic acid content in the phospholipids of human macrophages. Increased fatty acid desaturases 1 and 2 and acyl-CoA synthase long-chain family member 3 mRNA levels as well as increased arachidonic acid to linoleic acid and docosahexaenoic acid to eicosapentaenoic acid ratios were also found in atheroma plaque and peritoneal foam cells from LXR agonist-treated mice. By contrast, murine LXR-deficient macrophages displayed reduced expression of fatty acid elongase 5, acyl-CoA synthase long-chain family member 3 and fatty acid desaturases 1, as well as decreased cellular levels of docosahexaenoic acid and arachidonic acid. CONCLUSIONS: Our results indicate that LXR activation triggers PUFA synthesis in macrophages, which results in significant alterations in the macrophage lipid composition. Moreover, we demonstrate here that LXR agonist treatment modulates PUFA metabolism in atherosclerotic arteries.
2014
- ThesisCaractérisation de nouvelles cibles de LXR et impact sur le métabolisme lipidique et l’athéroscléroseAlexis VarinOct 2014
Les récepteurs nucléaires LXRα et LXRβ sont activés par la fixation de dérivés oxygénés du cholestérol. Ils régulent l’expression de nombreux gènes appartenant au métabolisme du cholestérol et des acides gras, et jouent un rôle important dans l’inflammation et l’immunité innée. L’activation de LXR inhibe le développement de l’athérosclérose, en augmentant l’efflux de cholestérol des macrophages ainsi que le transport inverse jusqu’au foie et l’excrétion biliaire. De plus, LXR diminue la biosynthèse et le captage du cholestérol dans les tissus périphériques. Enfin, les agonistes synthétiques de LXR administrés à des souris diminuent significativement l’inflammation dans les lésions athérosclérotiques, notamment en inhibant la sécrétion de certaines cytokines inflammatoires. Néanmoins LXR régule également la lipogenèse et la synthèse d’acides gras monoinsaturés, et l’administration d’agonistes de LXR s’accompagne également d’effets indésirables liés à cette régulation, comme une accumulation dérégulée d’acides gras dans le foie et une augmentation du taux de LDLs circulantes. Plusieurs autres mécanismes restent encore à être explorés, comme la synthèse d’acides gras polyinsaturés et les conséquences sur le métabolisme cellulaire. Nos travaux identifient une nouvelle voie régulée entièrement par LXR, le métabolisme des acides gras polyinsaturés. Le récepteur nucléaire LXR régule l’ensemble des enzymes FADS1, FADS2 et ELOVL5, responsables de la synthèse d’acides gras polyinsaturés oméga-6 et oméga-3. Cette régulation s’accompagne d’une incorporation d’acide arachidonique dans les phospholipides, via la régulation de LPCAT3, ce qui prépare les macrophages à une synthèse accrue de dérivés inflammatoires issus de l’acide arachidonique, comme la Prostaglandine E2, suite à une stimulation au lipopolysaccharide. La régulation de cette voie par LXR a également un effet sur le développement de l’athérosclérose, augmentant les taux d’acides gras polyinsaturés oméga-6 et oméga-3 dans les plaques d’athérome. Nos résultats montrent donc que LXR régule la synthèse des acides gras polyinsaturés en plus des acides gras mono-insaturés et de la lipogenèse et que cette régulation a des conséquences sur le profil lipidique des macrophages in vitro et in vivo ainsi que sur leur réponse inflammatoire.
2013
- Impact Factor 5-10Knock-down of the oxysterol receptor LXRα impairs cholesterol efflux in human primary macrophages: lack of compensation by LXRβ activationMinako Ishibashi, Rodolphe Filomenko, Cédric Rébé, Angélique Chevriaux, Alexis Varin, Valentin Derangère, Ginette Bessède, Philippe Gambert, Laurent Lagrost, and David MassonBiochem. Pharmacol., Jul 2013
Liver X Receptors (LXRs) αand βare oxysterol-activated nuclear receptors involved in the control of lipid metabolism and inflammation. Pharmacological activation of LXR is promising in the treatment of atherosclerosis since it can promote cholesterol efflux from macrophages and prevent foam cell formation. However, the development of LXR agonists has been limited by undesirable side-effects such as hepatic steatosis mediated by LXRαactivation. Therefore, it has been proposed that targeting LXRαactivators to extrahepatic tissues or using LXRβ-specific activators could be used as alternative strategies. It is not clear whether these molecules will retain the full atheroprotective potential of non-selective agonists. Our aim was therefore to determine the contribution of LXRαand LXRβto the control of cholesterol efflux in human macrophages. LXRαand/or LXRβexpression was suppressed by small interfering RNAs in human primary macrophages treated or not with synthetic LXRα/βdual agonists T0901317 and GW3965. We observed that LXRβsilencing had no detectable impact on the expression of LXR-target genes such as ABCA1 and ABCG1. Moreover it did not affect cholesterol efflux. In contrast, LXRαsilencing reduced the response of these LXR-target genes to LXR agonist and inhibited cholesterol efflux to ApoA-I, HDL2 or to endogenous ApoE. Importantly, no differences were observed between LXRαand LXRα/βknockdown conditions. Altogether, our data demonstrate that LXRβactivation is unable to maintain maximal cholesterol efflux capacities in human primary macrophages when LXRαexpression is impaired. In contrast to earlier mouse studies, LXRαlevels appear as a limiting factor for macrophage cholesterol efflux in humans.
- Impact Factor 5-10Liver x receptor regulates arachidonic acid distribution and eicosanoid release in human macrophages: a key role for lysophosphatidylcholine acyltransferase 3Minako Ishibashi*, Alexis Varin*, Rodolphe Filomenko, Tatiana Lopez, Anne Athias, Philippe Gambert, Denis Blache, Charles Thomas, Thomas Gautier, Laurent Lagrost, and David MassonArterioscler. Thromb. Vasc. Biol., Jun 2013
OBJECTIVE: Liver X receptors (LXRs) are oxysterol-activated nuclear receptors that are highly expressed in macrophages and regulate lipid homeostasis and inflammation. Among putative LXR target genes, lysophosphatidylcholine acyltransferase 3 (LPCAT3) involved in the Lands cycle controls the fatty acid composition at the sn-2 position of glycerophospholipids and, therefore, the availability of fatty acids, such as arachidonic acid (AA), used for eicosanoid synthesis. The aim of our study was to determine whether LXRs could regulate the Lands cycle in human macrophages, to assess the consequences in terms of lipid composition and inflammatory response, and to work out the relative contribution of LPCAT3 to the observed changes. APPROACH AND RESULTS: Transcriptomic analysis revealed that LPCAT3 was upregulated by LXR agonists in human macrophages. Accordingly, LXR stimulation significantly increased lysophospholipid acyltransferase activity catalyzed by LPCAT3. Lipidomic analysis demonstrated that LXR activation increased the AA content in the polar lipid fraction, specifically in phosphatidylcholines. The LXR-mediated effects on AA distribution were abolished by LPCAT3 silencing, and a redistribution of AA toward the neutral lipid fraction was observed in this context. Finally, we observed that preconditioning of human macrophages by LXR agonist treatment increased the release of arachidonate-derived eicosanoids, such as prostaglandin E2 and thromboxane after lipopolysaccharide stimulation, with a significant attenuation by LPCAT3 silencing. CONCLUSIONS: Altogether, our data demonstrate that the LXR-mediated induction of LPCAT3 primes human macrophages for subsequent eicosanoid secretion by increasing the pool of AA, which can be mobilized from phospholipids.
- Impact Factor < 5Biological activities of the LXRα and βagonist, 4β-hydroxycholesterol, and of its isomer, 4α-hydroxycholesterol, on oligodendrocytes: effects on cell growth and viability, oxidative and inflammatory statusThomas Nury, Mohammad Samadi, Alexis Varin, Tatiana Lopez, Amira Zarrouk, Mohamed Boumhras, Jean-Marc Riedinger, David Masson, Anne Vejux, and Gérard LizardBiochimie, Mar 2013
The biochemical and biological properties of 4β-hydroxycholesterol and of its isomer, 4α-hydroxycholesterol, are not well known. So, we determined the ability of 4α- and 4β-hydroxycholesterol to react with LXRαand LXRβ, and we characterized the activities of these oxysterols on oligodendrocytes which are myelin synthesizing cells. The effects of 4α- and 4β-hydroxycholesterol were studied on 158N murine oligodendrocytes to assess their activities on cell growth and viability, oxidative and inflammatory status. To this end different parameters were used: cell counting with trypan blue; identification of dead cells and cell cycle analysis with propidium iodide; evaluation of mitochondrial depolarization, lysosomal membrane integrity, actin depolimerization, nuclear morphology, and superoxide anion production after staining with JC-1, acridine orange, rhodamine-phalloidin, Hoechst 33342, and dihydroethidium, respectively; evaluation of ultrastructural changes by transmission electron microscopy, and cytokine quantification with a cytometric bead array. Only 4β-hydroxycholesterol is a LXRαand βagonist. No cytotoxic effects were found with 4α-hydroxycholesterol except a slight inhibition of cell growth at elevated concentrations. At high concentrations, 4β-hydroxycholesterol was not only able to inhibit cell growth, but also to induce cell death associated with a loss of mitochondrial transmembrane potential, dysfunctions of lysosomal membrane integrity, and superoxide anion overproduction. These side effects were lower than those observed with 7-ketocholesterol and 25-hydroxycholesterol used as positive controls. On oligodendrocyte murine primary cultures, only lysosomal membrane integrity was slightly affected under treatment with 4α- and 4β-hydroxycholesterol. So, 4α- and 4β-hydroxycholesterol have different biological activities. Their ability to induce cytotoxic effects on oligodendrocytes can be considered as weak comparatively to 7-ketocholesterol and 25-hydroxycholesterol.